The FDA’s Emergency Authorization of Booster Shots for 12 to 15-Year-Olds: A Call for Action
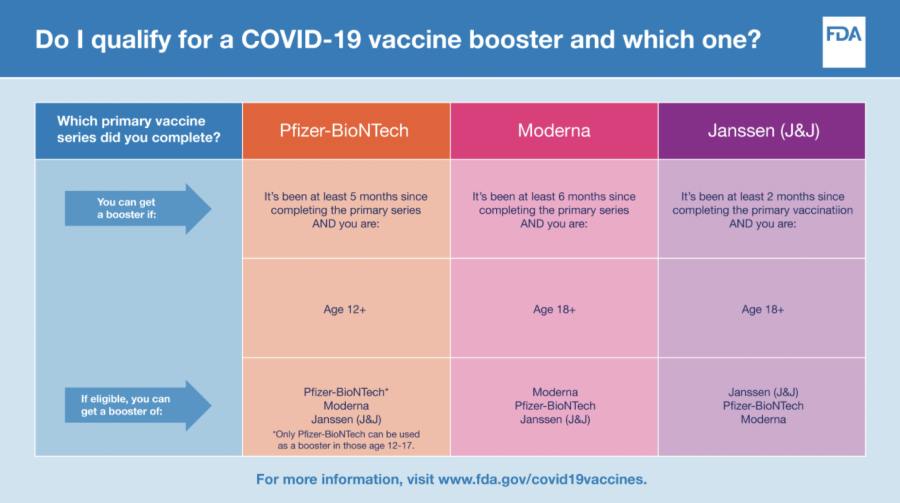
An FDA infographic explains the age requirements, timing, and brand of booster vaccination. The booster interval has been changed for those 12 years of age and older as of Jan. 3, a motion that impacts many students who may be at high exposure.
As of Jan. 3, the U.S. Food and Drug Administration (FDA) called for the authorization of the Pfizer-BioNTech COVID-19 Vaccine booster dose for those as young as 12-years-old and immunocompromised children ages 5 through 11. The period between two doses and the booster itself was shortened from six months to five months, an example of the urgency in an ever-changing pandemic.
People ages 12 to 15 were initially permitted to receive the Pfizer vaccine in May 2021, however, a rise in variants such as Delta and Omicron called for further precautions. Immunocompromised children, who are vulnerable to the virus due to health conditions or medications, have been taken into consideration when it comes to prioritizing booster dose distribution. The booster, which contains the same formulation as the initial COVID-19 vaccines, is becoming more widespread.
“These booster shots help reinvigorate your body to get better at that immune system response,” Biomedical Sciences teacher Juli Bachman said.
Questions on the booster shots’ side effects have been prevalent, however, the FDA’s research studies argue that there are rarely any safety concerns based on research studies for the newly-administered age group. As a matter of fact, not receiving the booster shot could become more detrimental to those who may be exposed to infection. Protection against variants, possible hospitalization and death are more likely to be combated after the third dose.
Junior Alisa Sumwalt has made plans to receive the booster dose.
“I’m vaccinated but I want to make sure that the effect of it is still there,” Sumwalt said. “If I get COVID, I don’t want it to be really bad so this would just be another level of protection.”
It has been reported by the CDC that the booster dose is 75% effective against the Omicron variant whereas the two initial shots are only 35% effective. An exact timeline of how long each dose of the COVID-19 vaccine remains effective is uncertain yet it is clear that over time, ensured protection from the virus decreases.
On Jan. 2, 315,000 new infections were reported in the U.S., serving as a wake-up call to Americans since these numbers are the highest seven-day average of COVID-19 cases at large. Life-saving, preventative measures have become even more essential.
Dr. Alessandro Sette of the La Jolla Institute for Immunology has been fundamental in the worldwide endeavor to understand SARS-CoV-2. Sette has found the immune response, either through prior infection or vaccination, to be crucial in producing antibodies to locate and neutralize the virus, therefore, preventing its advancement.
“[Vaccination] is one of the only things that can be done against Omicron apart from obviously using masks and socially distancing,” Sette said. “The vaccine will still protect against the disease and hospitalization, it is also reasonable to assume that the people that have been vaccinated may be spreading less virus and be potentially less infectious even though with Omicron that is still open to debate.”

Cellular immunity, the mediation of T cells into infected cells to shred the virus, is critical in containing the infection and is still being researched by Sette and his team. The function of T cells works hand-in-hand with antibodies and is nourished by the vaccine. According to Sette, variants may pass through the antibody response, however, the T cell response will not let it escape.
“If a variant like Omicron has many mutations… it will be less easy to neutralize the antibody response to prevent infection but if the T cells are still there then you could imagine that the infections would still be contained and severe disease would be avoided.”
⅓ of those vaccinated in the U.S. have received the booster shot thus far. Research experts argue that in order to achieve herd immunity, 80-90% of the population must be immune.
Appointments for either dose of the vaccine or the booster shot can be made at vaccines.gov. All Californians within age requirements qualify for free vaccination regardless of health insurance or immigration status.
“If something is approved for use, there is still the issue of if people are going to actually use it and that is something that is important to emphasize and perhaps an issue of outreach and advocating and helping generate that response,” Sette said. “I still wanted to reemphasize again that the large majority of hospitalized cases are non-vaccinated and non-boosted people.”